45 medication labels must include
Safety Enhancements Every Hospital Must Consider in Wake of ... Jan 17, 2019 · Place auxiliary labels on all storage locations and/or ADC pockets/ drawers/lids that contain neuromuscular blockers that clearly warn that respiratory paralysis will occur, and ventilation is required (e.g., “WARNING: CAUSES RESPIRATORY ARREST—PATIENT MUST BE VENTILATED”). The warning should be visible when ADC pockets/drawers/lids are open. Ch 8 Pharmacy Flashcards & Practice Test - Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber D. Name, address, and telephone number of the dispensing pharmacy Name, address, and telephone number of the prescriber
Medication package insert - Wikipedia In the United States, the Food and Drug Administration (FDA) determines the requirements for patient package inserts. In the United States, the FDA will occasionally issue revisions to previously approved package inserts, in much the same way as an auto manufacturer will issue recalls upon discovering a problem with a certain car.
Medication labels must include
Patient Labeling 101 - Food and Drug Administration Patient labeling should be written at a 6 to 8 th grade reading level z Use of certain fonts: Verdana, Arial, or APHont size 11 or greater for better visibility z Use of text boxes, bold font, and... US Food and Drug Administration's Requirements on Content and Format ... The regulation required that drug labels include information about the frequency and severity of adverse reactions to drugs, as well as the organs affected. It provided a fill-in-the-blank sentence with space for the name of a drug, the reported reactions, and the likelihood of a consumer experiencing an adverse reaction. 4. Documenting Medications (MAR). | Aplmed Academy Prescription labels will contain: ... (OTC) medications include in the original manufacturer's bottle with the resident's name, OR Labeled by the pharmacy; ... If the licensed practitioner changes the dosage on prescribed medication, a new prescription must be filled by the pharmacy. The old medication cannot be administered and must be ...
Medication labels must include. The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC... What Is a Drug Label? | The Motley Fool A drug label refers to all the printed information included with any dietary supplement, over-the-counter medicine, or prescription drug. They're strictly regulated by the Food and Drug... Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of... What's on a prescription label? - Knowledge is the best medicine In general your label will contain the following information:. Parts of a Prescription Label Rollover A-K below to see the various part of a prescription label. * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada.
FDA Says Drug Labels Must Include Clear Guidance for Pregnant Wom Starting June 30, new drug labels will have categories for "Pregnancy," "Lactation," and "Females and Males of Reproductive Potential." "Pregnancy" will include information on ... Medication Management - California Medication Labels (cont.) • Careful reading of the label is critical to ensuring medication safety. The information on the pharmacy medication label includes: Medication Management. 15. oPharmacy/pharmacist name, phone and address oPrescription number oIndividual’s name oPrescriber’s name (doctor) oName of Medication oStrength oDose The Pharmaceutics and Compounding Laboratory - Pharmlabs Prescription Product Labeling. Proper labeling is one of the most important aspects of dispensing a prescription. The label must comply with state and federal regulations and should correctly and clearly convey all necessary information regarding dosage, mode of administration, and proper storage of the product. Guidance Document: Labelling of Pharmaceutical Drugs for Human Use (1.4) When the composition of the drug varies from one lot to another, the outer label must include a reference to all non-medicinal ingredient alternatives that may be present in the drug, ... Claims on drug product labels that include market share, sale, consumer and patient use/ choice, or preference must be supported by adequate studies ...
Chapter 5: Prescriptions and Labels Flashcards | Quizlet Drug Labels Regulated by the Food and Drug Administration (FDA), which determines what needs to be on the label Dispensing pharmacist's label must include: Pharmacy name, address, and phone number Dispensing date Dispensing date may differ from the date on the prescription. Rx number, which identifies this unique prescription in the computer system FDA regulations require that all medication labels include taskmasters. All medication label should be approved by the FDA before a drug be released to the public. The label should include the active ingredient in the product, the use of the product either to treat or prevent, warnings, Inactive ingredients, purpose or the product action, directions or the dosage information. A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name Understanding Drug Labels | Basicmedical Key The epinephrine injection label ( Fig. 5.6) indicates a dosage supply of 0.1 mg/mL, and the total volume of the ampul is 10 mL. Figure 5.4 The dosage strength of this dosage form of Diflucan ® (fluconazole) is 200 mg. Figure 5.5 The dosage strength of this drug is 125 mg (200,000 units) penicillin V in 5 mL.
Medication Administration Safety - Patient ... - NCBI Bookshelf There is a large and growing body of research addressing medication safety in health care. This literature covers the extent of the problem of medication errors and adverse drug events, the phases of the medication-use process vulnerable to error, and the threats all of this poses for patients. As this body of literature is evaluated, the fact that there are crucial areas about which we know ...
Guidance for Industry - Food and Drug Administration 78 The format and content of prescription drug and biological product labels and labeling must 79 comply with FDA regulations in 21 CFR part 201 for drugs and 21 CFR part 610 Subpart G-
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's...
How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine.
Pharmaceutical Labeling: Requirements & Guidelines To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
OTC Labeling Requirements - FindLaw The FDA conducted two studies to evaluate the effects these parameters have on the drug label readability, and after evaluating the studies concluded that a minimum type size of 6.7point is required to ensure that people over the age of 60 can read the label. Recognizing the space constraints, however, the FDA chose to allow type size as small ...
PDF Labeling on the Sterile Field: Improve Patient Safety and Ensure Joint ... Labeling must include: Name of medication or solution, strength, date, and time Label one item at a time. Single items must also be labeled.
How to Read Over-the-Counter and Prescription Drug Labels Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose.
Over the Counter (OTC) Drug Labels - Poison This is the easiest way to prevent errors and overdoses, because OTC medicines are often used without health professional advice. All over-the-counter (OTC) medication labels contain Drug Facts. Drug Facts include important information about the active ingredient (s), uses, warnings, doses, and directions. Serkalem Mekonnen, RN, BSN, MPH.
Medication - Wikipedia A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. [1] [2] Drug therapy ( pharmacotherapy ) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate ...
Drug labeling, Information about Drug labeling - FAQs Each product must contain a label with "Supplement Facts" in bold letters onthe front panel. This is the manufacturer's opportunity to identify the product. Below "Supplement Facts," the panel must state the serving size. This isdetermined by the manufacturer with no input from the FDA.
PDF Chapter 20 Labeling Medications and Expiration Dating someone other than person administering, must also include patient name and location, directions for use, and auxiliary labels c. Other labeling considerations: ... NPSG.03.04.01: Label all medications, medication containers, and other solutions on and off the sterile field in perioperative and other procedural settings
Safe Labeling Helps Prevent OR Medication Errors Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared.
Safety Considerations for Container Labels and Carton ... In this guidance, all such products are jointly referred to as products, drugs, or drug products unless otherwise specified, and persons responsible for designing product container labels and






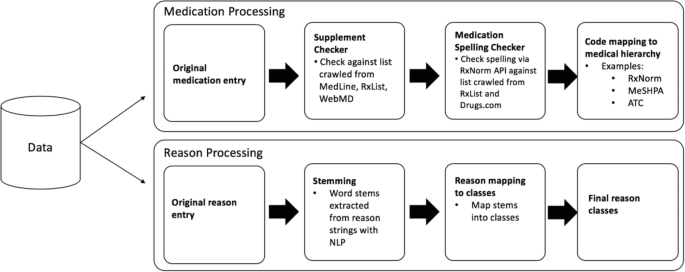

Post a Comment for "45 medication labels must include"